Symbols Glossary
View common symbols, references, titles, and explanations of symbols below.
|
SYMBOL |
STANDARD REFERENCE |
STANDARD TITLE |
SYMBOL TITLE |
EXPLANATORY TEXT |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Manufacturer |
Indicates the medical device manufacturer |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Authorized Representative in the European Community |
Indicates the authorized representative in the European Community / European Union |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Date of manufacture |
Indicates the date when the medical device was manufactured |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Use-by date |
Indicates the date after which the medical device is not to be used |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Batch code |
Indicates the manufacturer's batch code so that the batch or lot can be identified |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Catalogue number |
Indicates the manufacturer's catalog number so that the medical device can be identified |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Serial number |
Indicates the manufacturer's serial number so that a specific medical device can be identified |
 |
ISO 15223- 1:2020(E) DRAFT |
Medical devices — Symbols to be used with medical device labels, labeling and information to be supplied |
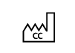
Country of manufacture |
To identify the country of manufacture of products |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Non-sterile |
Indicates a medical device that has not been subjected to a sterilization process |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Fragile, handle with care |
Indicates a medical device that can be broken or damaged if not handled carefully |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
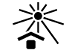
Keep away from sunlight |
Indicates a medical device that needs protection from light sources |
 |
ISO 15223- 1:2016 and ISO 15223-1:2020(E) DRAFT |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Keep dry |
Indicates a medical device that needs protection from moisture |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Lower limit of temperature |
Indicates the lower limit of temperature to which the medical device can be safely exposed |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Upper limit of temperature |
Indicates the upper limit of temperature to which the medical device can be safely exposed |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Temperature limit |
Indicates the temperature limits to which the medical device can be safely exposed |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
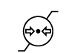
Humidity limitation |
Indicates the range of humidity t which the medical device can be safely exposed |
 |
ISO |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part |
Atmospheric pressure limitation |
To indicate the acceptable upper and lower limits of atmospheric pressure for transport and storage. |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Do not use if package is damaged |
Indicates a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information |
 |
ISO 15223-1:2016E |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Do not re-use |
Indicates a medical device that is intended for one single use only |
 |
ISO 15223-1:2016 and ISO 15223-1:2020 Reference no. 5.4.3. (ISO 7000-1641) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Consult instructions for use |
Indicates the need for the user to consult the instructions for use |
 |
ISO 15223-1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Consult instructions for use or consult electronic instructions for use |
Indicates consult instructions for |
 |
ISO 15223- 1:2016 Reference no. 5.4.4. (ISO 7000-0434A) |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
Caution |
To indicate that caution is necessary when operating the device or control close to where the symbol is placed, or to indicate that the current situation needs operator awareness or operator action in order to avoid undesirable consequences |
 |
iso_grs_7010_WOO1 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
General warning sign |
To signify a general warning |
 |
ISO 15223- 1:2016 |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied – Part 1: General requirements. |
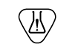
Contains or presence of natural rubber latex |
Indicates the presence of dry natural rubber or natural rubber latex as a material of construction within the medical device or the packaging of a medical device |
 |
ISO/DIS 15223- 1:2020(E) DRAFT |
Medical Devices — Symbols to be used with medical device labels, labeling, and information to be supplied — Part 1: General requirements. (Draft of New Version) |
Contains hazardous substances |
Indicates a medical device that contains substances that can be carcinogenic, mutagenic, reprotoxic (CMR), or substances with endocrine-disrupting properties |
 |
ISO/DIS 15223- 1:2020(E) DRAFT |
Medical Devices — Symbols to be used with medical device labels, labeling, and information to be supplied — Part 1: General requirements. (Draft of New Version) |
Medical device |
Indicates the item is a medical device |
 |
ISO 15223-1:2020(E) DRAFT |
Medical devices — Symbols to be used with medical device labels, labeling, and information to be supplied — Part 1: General requirements. |
Translation |
To identify that the original medical device information has undergone a translation which supplements or replaces the original information |
 |
EC 60601-1, Reference no. Table D.2, Safety sign 10 (ISO 7010-M002) |
Medical electrical equipment — Part 1: General requirements. for basic safety and essential performance |
Refer to instruction manual/booklet |
To signify that the instruction manual/booklet must be read |
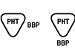 |
BS EN 15986:2011 |
Symbol for use in the labeling of medical devices — Requirements for labeling of medical devices containing phthalates |
Contains or presence of phthalate: benzyl butyl phthalate (BIPingreBP) |
Medical device is derived from or manufactured from products containing phthalate: benzyl butyl phthalate (BBP) |
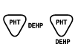 |
BS EN 15986:2011 |
Symbol for use in the labeling of medical devices — Requirements for labeling of medical devices containing phthalates |
Contains or presence of phthalate: bis (2- ethylhexyl) phthalate (DEHP) |
Medical device is derived from or manufactured from products containing phthalate: bis (2- ethylhexyl) phthalate (DEHP) |
 |
BS EN 15986:2011 |
Symbol for use in the labeling of medical devices — Requirements for labeling of medical devices containing phthalates |
Contains or presence of phthalate: combination of bis (2-ethylhexyl) phthalate (DEHP) and benzyl butyl phthalate (BBP) |
Medical device is derived from or manufactured from products containing bis (2-ethylhexyl) phthalate (DEHP) and benzyl butyl phthalate (BBP) |
 |
BS EN 15986 |
Symbol for use in the labeling of medical devices — Requirements for labeling of medical devices containing phthalates. |
Negation symbol plus Presence of phthalate symbol, together meaning free of phthalates |
Manufacturers wishing to communicate the meaning “does not” or “is not” where a symbol expressing this meaning does not exist, should follow the method set out in EN 80416- 3:2002, Clause 7 |
 |
ISO 7000 |
Graphical symbols for use on equipment. |
Packaging unit |
To indicate the number of pieces in the package |
 |
ISO 7000 |
Graphical symbols for use on equipment - registered symbols |
This way up |
N/A |
 |
ISO 7000 |
Graphical symbols for use on equipment-Registered symbols |
Do not stack |
To indicate that the item shall not be vertically stacked, either because of the nature of the transport packaging or because of the nature of the items themselves |
 |
ASTM F2503 |
Standard practice for Making Medical Devices and other item for safety in the magnetic resonance environment |
Magnetic Resonance (MR) safe |
3.1.13: An item that poses no known hazards resulting from exposure to any MR environment. MR Safe items are composed of materials that are electrically nonconductive, nonmetallic, and nonmagnetic. |
 |
ASTM F2503 |
Standard Practice for Marking Medical Devices and Other Items for Safety in the magnetic resonance environment. |
MR Conditional |
3.3.1.11: an item with demonstrated safety in the MR environment within defined conditions including conditions for the static magnetic field, the time-varying gradient magnetic fields and the radio frequency fields. |
 |
ASTM F2503 |
Standard Practice for Marking Medical Devices and other Items for safety in the Magnetic Resonance Environment |
(MR) Unsafe |
3.1.14: An item which poses unacceptable risks to the patient, medical staff or other persons within the MR environment |
 |
EU 2017-745 EU 2017-746 |
REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017 on medical devices, amending Directive 2001/83/ EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/ EEC and 93/42/EEC |
CE marking |
(43) ‘CE marking of conformity’ or ‘CE marking’ means a marking by which a manufacturer indicates that a device is in conformity with the applicable requirements set out in this Regulation and other applicable Union harmonization legislation providing for its affixing |
 |
GHS |
Globally Harmonized System of Classification and Labeling of Chemicals (GHS), Eighth Revised Edition |
Highly flammable |
Medical device contains materials that are highly flammable. Appropriate caution should be taken |
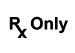 |
N/A |
N/A |
Prescription Use Only |
Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner. |
 |
N/A |
N/A |
Do not freeze |
Indicates the medical device should not be frozen |
 |
N/A |
N/A |
Canadian and US Certification mark |
Products bearing this mark have been tested and certified in accordance with applicable US and Canadian electrical safety and performance standards |
 |
N/A |
N/A |
USA |
Manufactured in the USA and/or applies to medical devices sold in the USA |
 |
ISO 7001 |
Public Information Symbol |
Recycling |
To indicate the location of a recycling bin or container. |
 |
ISO 15223-1:2021 Reference no. 5.2.6. (ISO 7000-2608) |
Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. |
Do not resterilize |
Indicates a medical device that is not to be resterilized. |
|
ISO 15223-1:2021 Reference no. 5.2.3. (ISO 7000-2501) |
Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. |
Sterilized by ethylene oxide treatment |
Indicates a medical device that has been sterilized using ethylene oxide. |
|
|
ISO 15223-1:2021 Reference no. 5.2.4. (ISO 7000-2502) |
Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. |
Sterilized using irradiation |
Indicates a medical device that has been sterilized using irradiation. |
|
|
ISO15223-1: 2021 Reference no. 5.7.10 |
Medical devices — Symbols to be used with information to be supplied by the manufacturer - Part 1: General requirements. |
Unique device identifier
|
Indicates a carrier that contains unique device identifier information. |
